Intro
After trying to calibrate the conductivity of water with a coqui I decided to make a try in the field. Then go to the Suquía river, one of the most polluted rivers in central Argentina, but I chose a relatively low polluted site called Isla de los Patos (Duck’s island). I used a Coqui with a 10 kohm resistor and a 10uf capacitor. After seeing some issues with the calibration of Coqui I remembered that the TDS (total dissolved solids, are directly related with conductivity) of water in that site was in the range of best linearity (0.1-1g/L) of the calibration. Then the goal of that trip was to see if Coqui gives some accurate data, using as control a TDS meter.
Materials
- Coqui
- Commercial TDS meter
- Mobile phone or some device to record audio
- Glass to collect water
- Something to take notes
- Audacity software
Study site
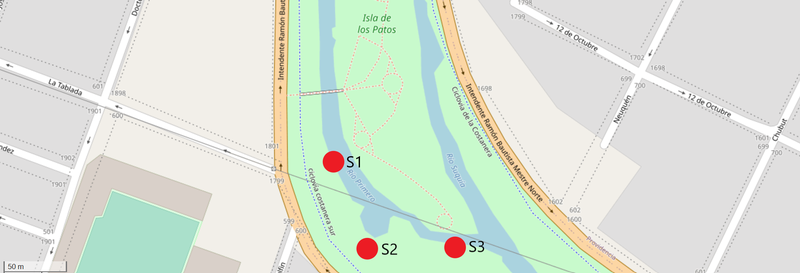
The chosen place for the study was the Isla de los Patos (Duck’s island) in the Suquía river. This site was previously studied in a Case of Study Note, with 3 points of sampling, upstream of a suspicious drainage (S1), the suspicious drainage itself (S2) and downstream of the suspicious drainage (S3).
Results
The good news 😃
The relationship between TDS and distance between peaks of sound produced by Coqui (as explained in a previous note) was really linear!!! That is so good for the reliability of the Coqui measurements.
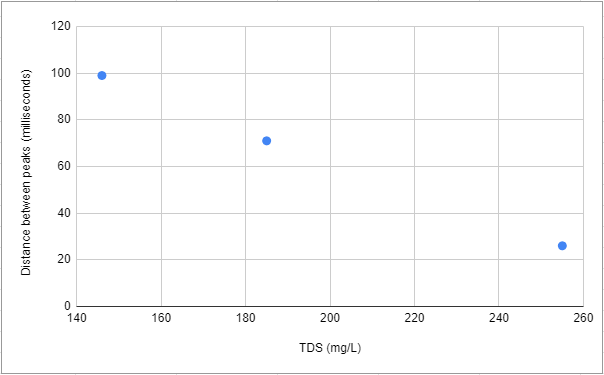
The bad news 😟
The relationship between TDS and distance between peaks was inversely proportional. That is strange because in the previous calibration note the relationship was directly proportional. Is noteworthy that the water temperature was not the same and the difference between samples in TDS values was smaller than the one used for the calibration curve.
Some thoughts 💭
It will be interesting to calibrate the Coqui for smaller ranges and with more points that were used for the previous calibration note. Also, it will be interesting to take in consideration the effect of temperature in conductivity.

9 Comments
It looks like the Coqui is a pretty well designed instrument. But, some of the pharmaceutical instruments would send the tone through a bridge. The top two legs of the bridge were just resistors. The bottom leg on one side sent the tone through a calibrated cell ( the constant varied depending on the purity of the water). The other side went to a calibrated set of resistors. Between the two legs of the bridge was a meter(-,0,+). The idea was to put the cell in the solution and adjust the resistance until the meter read zero. Its rather old school. But maybe the bridge part would help out?
Is this a question? Click here to post it to the Questions page.
Thanks for your suggestion @Ag8n, sounds very interesting to try. Anyway I have to be honest and say that I don't quite figure out how to do what you suggest. Is there a bibliography that I can read to better understand what you are proposing? Some diagram maybe could help Thanks for your suggestion, sounds very interesting to try. Anyway I have to be honest and say that I don't quite figure out how to do what you suggest. Is there a bibliography that I can read to better understand what you are proposing? Some diagram maybe could help
Is this a question? Click here to post it to the Questions page.
Look up conductivity cell and wheatstone bridge Instead of feeding DC into the weatstone bridge, the audio signal is fed in. One leg of the wheatsone bridge is a liquid conductivity cell. The other side is a variable resistor that you adjust until the meter or sound "nulls"(reaches aminimum). One of the EPA methods (EPA method 120.1:Conductance(specific conductance, umhos at 25C) by Conductivity Meter, gives details on using a Wheatstone bridge type device. I looked them up on the internet an they are still listed by a number of suppliers. Basically, its an audio generator feeding a wheatsone bridge. The old instrument we had required platinum electrodes for use with high purity DI water. We would test how contaminated washed devices would change water conductivity. But there is no reason platinum electrodes should be needed for this work. Therre has to be a cheap way to do this. I jsut dont know what it is.
Hi @Ag8n! What you suggest sounds interesting. Would it be something similar to the attached diagram?
Is this a question? Click here to post it to the Questions page.
The conductivity cell should take the place of Rx. So disconnect the conductivity cell, disconnect Rx, leave the one of the conductivity cell connected as it is, and move the end that's connected to the intersection of r1 and r2, and move it to the other end of r2 (the same one that goes to the coqui). The detector needs to be the bridge that goes between the the intersection of r1 and r2 ( connection 1) and r3 and the conductivity cell ( connection 2). The trick is- the detector needs to be high impedance. This can be done with a number of meters- but you are detecting voltage at about 3000 hz. Which is a bit of a trick. You are also looking for a null, or zero.
Reply to this comment...
Log in to comment
This is very cool! I do wonder if TDS is going to be consistently correlated with conductivity -- i may just not be well informed but isn't it possible that the dissolved solids are not conductive, or are all soluble solids conductive? Like, could you dissolve something electrically inert -- a pigment? -- that doesn't affect conductivity? Or is high TDS always conductive?
Related: does the TDS sensor use an optical measurement or how does it work? Could you compare the 3 samples using a commercial conductivity sensor?
Sorry one more question! How far apart were the temperatures? Just a couple degrees, or very different?
Thank you! Great note!
Is this a question? Click here to post it to the Questions page.
Especially in mixtures, not all soluble solids are conductive. So yes, you can dissolve (or sometimes suspend) something inert in water. We would usually do at least a couple of tests to make sure we got most things. The other was usually total solids. And TDS and conductivity don't always directly relate. Was reading premierwatermn.com/water-quality/water-contaminants/total-dissolved-solids/. It said, "TDS is calculated by converting the EC by a factor of 0.5 to 1.0 times the EC, depending on the levels." As for how the TDS work.... The above quote makes you think it's done with EC, but don't know.
Typically, all the conductivity testing we did was close to room temp, although that was measured by the instrument.
The major difference in EC and TDS probably explains many of the differences (the 0.5 to 1.0 factor).
Thanks @Ag8n for the explanation! I am learning a lot! 🤓
Reply to this comment...
Log in to comment
Further note-EC is electrical conductivity
Reply to this comment...
Log in to comment
Login to comment.