I'm very curious about lead detection with deep UV - basically using UV in the 200-300nm range to make lead visibly glow in the visible range.
I don't see the technique listed on this page. But doing some deeper research...
A long time ago I used a toothbrush sterilizer to try to see lead residue on a bullet casing and it didn't glow at all. But I do see the references (https://libanswers.cmog.org/faq/143932) to "icy blue" fluorescence in glass due to lead:
Pb (Lead) -- A strong icy-blue response, but not normally as strong as U. The fluorescence is visible under both long-wave and short-wave UV. High-lead glasses are usually colorless. The fluorescence becomes noticeable at a level of about 5%, and is strong by about 10-15%. Pb normally fluoresces more strongly than manganese or antimony. (Don't mistake the reflection of visible purple light from the UV lamp for Pb fluorescence. The Pb fluorescence is an icy-blue color and emanates from within the body of the glass.)
This page: https://www.uvminerals.org/minerals/common-fluorescent-minerals/ notes:
Cerussite is a lead carbonate, and interestingly, lead is often an actuator for fluorescence in mineral specimens
and
not all specimens of these minerals will fluoresce, as minerals typically require an ion actuator to glow
So it seems like metallic lead may not fluoresce. That said, lead dissolved in water seems to usually be an ion Pb2+...
Basically i'm a little out of my depth on the chemistry and physics of fluorescence at this point. I'm seeing articles like these talk about the use of nanodots or similar substances to make lead and copper ions fluoresce. That suggests to me that they don't on their own. So maybe I'm just barking up the wrong tree here.
- https://www.researchgate.net/publication/337680338_Schiff_Base_Derived_from_44'-methylenedianiline_and_p-anisaldehyde_Colorimetric_Sensor_for_Cu2_Paper_Strip_Sensor_for_Al3_and_Fluorescent_Sensor_for_Pb2
- https://pubmed.ncbi.nlm.nih.gov/23369297/
The glass example mentioned at top does seem promising. Maybe it's worth shining some of these 254nm range LEDs at some older glassware. But the kind of simple detection I'd hope for would be something like this using deep enough UV and I just don't know enough to know if it's possible without a reagent:
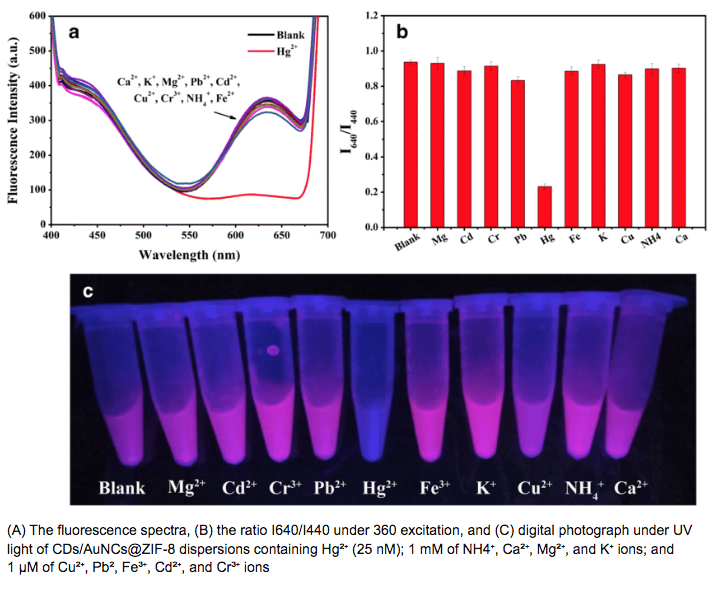
The last paper mentioned above is(I think) using chemiluminesence to generate the light(Flouescent detection of lead in... ByYan- Shiuan Wu, Et al.).The h2o2 is the first clue. The second one is the Amplex ultra red. These are reagents made by Thermo for chemiluminescence. More to come.
Reply to this comment...
Log in to comment
The first article from" research gate "is a little more interesting. It has lead forming something similar to chelates. The summary of the article was only available, but it had chemical structures drawn. It maybe fluorescing, but it is probably mostly due to the compound "L" (a mixture of methylenedianiline and anisaldehyde).
Reply to this comment...
Log in to comment
I worked a lot on lead with atomic aborpion(AA) and related techniques. The two lead lines we normally used were 283 nm and 214 nm. Of course, these aren't visible. One technique that we briefly studied in college, but I've never seen used, is atomic fluorescence spectroscopy. I looked it up and saw all kinds of crazy set ups from easy to wild. Thought I'd throw that in as a last thought. It might be worth looking into.
Interesting. It's like a high power version of this?
https://www.ebay.com/itm/324819370181
Does this take high voltage to run?
Is this a question? Click here to post it to the Questions page.
https://www.sciencedirect.com/science/article/abs/pii/0039914070800819
That looks like a hollow cathode lamp. We used perkin elmer, not fisher, so there could be differences. The lamp should have the element it can detect stamped on it. There are also multi-element lamps, in which case you will have several elements in the label. That is, you could analyze several elements with the same lamp. The operating current is listed at the bottom. SOP was always to go with the lowest current that would give good results. As the lamp aged, the current would need to be increased. We were told the voltage was high, but never the exact level. To give an idea, the first AA we had, used neon as the other gas in the hollow cathode lamp. It made alignment easier (you would get a neon red beam to align with). But that suggests that the voltage is at least 90 volts,if not higher.
Reply to this comment...
Log in to comment
That looks like a hollow cathode lamp. But there are other kinds. It should have the element on the base plus usually the operating current range. Go with the minimum current needed. As the lamps get older, the minimum current needed goes up. The amount of life ( as in operating hours) in the lamp is not huge. Some of them have a running time meter on the side to let you know how much time they have been in use. I think you want to stay away from these if possible. One of the papers from 1979 showed using a microwave, instead. By the way, I listed the two lead lines as 283 nm and 214 nm. We used 214 nm for Zn. If needed we would look for an alternate line around 217 nm. For Pb. My bad.
Reply to this comment...
Log in to comment
Aanalyst (a model of atomic absorption spectrometer that we had)hollow cathode lamps new are running for about $350. So when there are hollow cathode lamps advertised on ebay for $20, there's probably something wrong. It's usually that the hollow cathode lamp is nearly dead. If they have a running time meter on the hollow cathode lamp, And it shows low running time,it might be worth the gamble. Otherwise....
Reply to this comment...
Log in to comment
You know, getting a couple of these that could be "disposable" might not be a bad idea. Working with 400 volts isn't a terribly high voltage, but it's still high enough to let the smoke out of parts (i.e. Kill them). It looks like the operating voltage is about 400V. I'm old school and would try it with either a step up transformer or a 120v transformer and a voltage multiplier. High voltage Electrolytic capacitors are getting kind of hard to find these days. But there are ways around it. Caps in series with resistors across them. Sorry, I'm old school. How is the new approach to the problem? Thanks and regards.
Is this a question? Click here to post it to the Questions page.
Hmm... I guess it seems like a tough setup for my skill set. What does the microwave version look like?
Is this a question? Click here to post it to the Questions page.
Reply to this comment...
Log in to comment
Here are a couple of reference: "Studies in atomic fluorescence spectroscopy -III:microwave excited electrodeless discharge tubes as spectral sources for atomic fluorescence and atomic abstinence spectrometry" by R.M.Darnell et. al. Talanta, volume 14 issue 5, may 1967, pages 551-555
Reply to this comment...
Log in to comment
Another reference. "Some recent developments in atomic fluorescence spectrosopy" by T.S.West Analytical Chemistry, ( presented at 26 th international Congress of pure and applied chemistry , Tokyo, Japan, 4-10 Sept 1977), 1979 pages 837-843.
Reply to this comment...
Log in to comment
We also used graphite furnace AA, also called GFAA. One common analytical instrument is ICP (inductively coupled plasma). I've used them a couple of times. The instruments were large, at the time 4ft x 4ft x 4 ft ( that's been a while ago, so I'm sure they are smaller now) and used a lot and power. The company I was visiting had a special power line just for the ICP, because of the high power requirements. There are two common kinds of detectors, optical emission ( OE) and mass spec (MS). That's been a while ago, so the instrument size and power requirements should be lower, now. That's just to give an idea of what some of the instruments can use. The GFAA used a 220 line, was much less expensive and smaller than the ICP, but didn't have the same resolution. Oh well.
Thank you for the links and the explanations!!!
Reply to this comment...
Log in to comment