I am using this DFRobot Turbidity sensor. The associated wiki page has some sample code that includes some sample code and some information about converting between voltage and NTU (Nephelometric Turbidity Unit)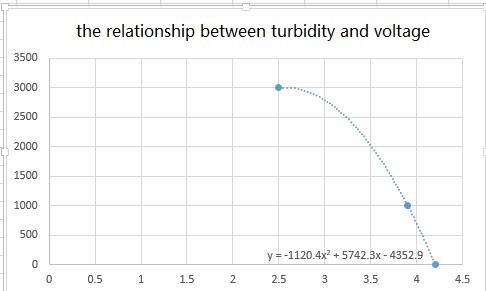
But I would like to verify this relationship and explore how various factors effect its accuracy. To do that, I would like to have some way to calibrate these sensors.
It appears that distilled water should have a turbidity of less than .1 NTU. It appears that you can purchase Formazin solution for turbidity calibration that comes in a standard 4,000 NTU solution.
Instructions that I have found on the internet say that you take this solution and "dilute it to the desired instrument range."
Is turbidity going to decrease linearly as I dilute this solution? So if I make a preparation of 1 part Formazin solution and 1 part distilled water, should it have a turbidity of 2,000 NTU?
Is this a good way to go about this calibration and testing? Are there other techniques I should look at?

Looks like there is an issue with reply by email, or possibly the list => email system, but Alan Marchiori responded on the
plots-waterqualitylist with:Is this a question? Click here to post it to the Questions page.
Reply to this comment...
Log in to comment
Hi wmacfarl ,
I've been thinking about affordable ways to calibrate diy photometric devices without having to use commercial standards like formazin or polymer suspensions.
It is very important that the calibration technique is accessible so that it can be reproduced by others using other instruments so that the results can be compared.
An alternative would be to express the readings always with reference to distilled water readings (a more accessible material): Abs (sample) / Abs (distilled water)
But found an interesting tip in the paper: https://www.sciencedirect.com/science/article/pii/S2468067218300762
The authors suggest the use of neutral filters, widely used in photography.
These filters have known transmittance and could be used to calibrate DIY photometers.
I haven't read yet all the paper in detail, but I intend to evaluate this alternative to the photometer I'm mounting.
I hope it helps.
Best regards, Markos
Reply to this comment...
Log in to comment
Hi wmacfarl, Some more info.
This type of sensor does not measure turbidity exactly.
It measures the absorbance, or attenuation of light when passing through the sample.
To measure turbidity the detector must be at an angle of 90 degrees to the source.
In turbidity what is measured is the light scattered by the particles, or microbubbles, suspended in the sample.
When the detector is aligned with the light source what is being measured is the aborbance, ie the attenuation in the light signal.
And aborbance (attenuation in light intensity) is the combined (summation) result of light that has been absorbed by the dissolved substances + the light that has been scattered by the particles that are in suspension.
Best regards, Markos
Reply to this comment...
Log in to comment
Hi wmacfarl,
Typically you would want to have some idea of the turbidity of whatever unknown sample you want to measure. If your measuring a freshwater stream, your maximum turbidity will probably be somewhere around 100 NTU. Once you have an idea of the maximum turbidity expected, then you should make your calibration solutions just beyond that limit (i.e. start with a solution around 150 NTU if you expect 100 NTU as your max). Once you have your maximum concentration solution, then you should make a series of more dilute solutions, including a pure water solution. This is usually done by taking some of you most concentrated solution and cutting it in half, by dilution, and repeating this process three or four times (i.e. you start with 100 NTU, then you would make 50 NTU, then 25 NTU...).
If you know your sensor has a linear response over the turbidity concentration range you are calibrating, then you can skip the serial dilution and only measure the high concentration sample and pure water sample in order to generate your calibration curve. Typically you would expect a linear response in lower turbidity concentrations (maybe less then 50 NTU). At higher turbidity concentrations the linearity will break down.
If you want to go down the path Markos is looking at (using neutral filters or something similar), I would suggest that you use those as a secondary standard. So you would first calibrate you instrument using NTU standards, then measure your secondary standard (neutral filter or the like) to get a NTU equivalent value for that standard. After that you can do without the NTU standards and only use the secondary standard.
Reply to this comment...
Log in to comment