The pH or hydrogen potential of a solution is a relevant variable when it comes to determining the quality of water, whether it is a drink or an aquatic environment. There are several proprietary commercial tools of varying complexity and precision to measure this parameter. However, for this activity we intend to use natural pigments that are sensitive to this parameter and thus be able to measure pH with elements that are available to anyone.
An environmental friendly method exist for pH determination of water samples. The Brasica oleracea cabage has high concentration of anthocyanins pigments that are sensitive to pH changes of a solution. This fact has been documented in several reseach papers that explore it potencial:
- https://www.sciencedirect.com/science/article/abs/pii/S0925400512014037
- https://www.sciencedirect.com/science/article/abs/pii/S0308814616314339
Purpose
Determine the pH of different samples with pigments extracted from purple cabbage (Brassica oleracea).
Necessary materials 📝
- A cup of chopped purple cabbage
- A cup of distilled water
- A clean pan
- Clean glass containers
- Samples of liquids of known pH (vinegar, sodium bicarbonate solution, bleach, laundry soap, carbonated cola drink).
- Sample of water that you are interested in knowing its pH. It can be from a nearby river or lake.
All steps in one
There is a YouTube link
Step 1 🔪
Cut the cabbage into small pieces with a knife to fill a cup
Step 2 🔥🔥🔥
Put the chopped cabbage with a cup of water in the saucepan and simmer for 15 minutes
Step 3 ❄️
Filter the liquid from the pot and let it rest until cool
Step 4 💧 ❓
Try different substances to find out their pH values. The image below indicates the equivalence between different colors and pH values.
 Making an indicator from red cabagge 🌈 by Andy Brunning / compoundchem.com; under licence CC BY-NC-ND 2.0
Making an indicator from red cabagge 🌈 by Andy Brunning / compoundchem.com; under licence CC BY-NC-ND 2.0
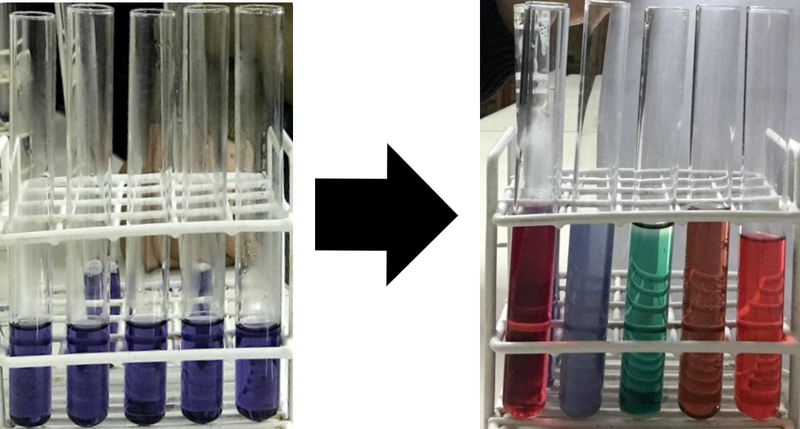 The test tubes above correspond to hydrochloric acid (dangerous), river water, sodium bicarbonate solution, carbonated cola drink and alcohol vinegar.
Original picture
The test tubes above correspond to hydrochloric acid (dangerous), river water, sodium bicarbonate solution, carbonated cola drink and alcohol vinegar.
Original picture
Step 6 📷
Share your photos with the PublicLab community and social media!!!
Wrap up
Knowing as much as we can about the aquatic environments that care us is key to being able to detect any negative effects on them in time.
Clarification: that the pH value of an environment is at normal values does not mean that this environment is not suffering some kind of disturbance, to determine that an environment is in optimal conditions requires a integrative analysis of it.

11 Comments
First, I'd like to thank Alejo for posting this page. It is very helpful. Then, I' d like to refer you to the comments page in compound chem ( dated May 18,2017 from the author of the page),for this procedure. One of the comments was that the indicators tendeded to "go off" after a while. The author responded by saying he extended the time by refrigerating the indicator solution. Another comment mentioned freezing the solutions into Ice cubes to extend life. The first comment( about the solutions going off after a short period of time) unfortunately matches my experience. In my case, the time was a few days. In industry, pure indicator solutions usually are dissolved in alcohol. I don't know if adding some alcohol to these solutions would extend their life or not.
Reply to this comment...
Log in to comment
Hi @Ag8n! Thanks for your reply! I had this cabbage solution in fridge almost a week and I didn't see an appreciable color change. I don't know if alcohol could be a good preservant, I'll would try it.
Reply to this comment...
Log in to comment
I did this! We tested vinegar, baking soda, lemon juice, pickle juice, and carbonated water. A lot of acids easily available in the kitchen. Then we mixed it all together at the end 😃
Reply to this comment...
Log in to comment
For a couple other reads, look up "The Effect of pH on Color Behaviour of Brassica oleracea Anthocyanin" in Journal of Applied Sciencies, volume 11(13):2406-2410,2411. They use a colorimeter based on human vision, so the coordinate system may not be familiar. But it is valid. Another quick and simple prep method is scienceprojectlab.com/red-cabbage-indicator.html. I was hoping these could be used as titration indicators. That usually means storing in the solution phase for at least a month. The liquid form does not appear to be stable. Although the"Applied Science" paper did have a dried form ( probably purchased).
Reply to this comment...
Log in to comment
Interesting paper on this same subject. " Application of Butterfly Pea extract as an indicator of acid - base titrations " by Nyi Mekar Saptarini et. al., Journal of Chemical and Pharmaceutical Research 2015 7(2):275-280. It also used water extractions and gives very good information on using these flowers for titrations. No expiration date is given for the solution(not a good sign).
Reply to this comment...
Log in to comment
Some natural indicators can have shelf lives of up to 90 days in solution. See "Hippeastrum hybridum anthrocyanin indicators of acid - base titrations" by Robert Byamukama et. al., Int. J. Biol. Chem. Sci. 10(6): 2716-2727 Dec 2016
Reply to this comment...
Log in to comment
Check this reference: "Evaluation of red cabbage dye as a potential natural color for pharmaceutical use" by Neela Chigurupati, et. al. Industrial Journal of Pharmaceutics, 241(2002)293-299.
You need to go to section 3.4 "Stability of red cabbage solutions in water". To quote "the dye was most stable at room temperature and pH3(percentage of degradation was only 1-5% over a period of 10 days). However, it was least stable at 50C and pH 8(percentage of degradation was 79-98% over a period of 10days)." Section 3 .5 had some interesting info on using it as an indicator, as well. So, at the very least, the cabbage indicator needs to be stored in acidic media.
Reply to this comment...
Log in to comment
Clarification. Acidic media is pH 3.
Reply to this comment...
Log in to comment
The acid (from the pH 3 solution ) for the cabbage indicator could mess up some titrations. A blank might need run(and, depending on the titration, the blank could be substantial). A better approach might be freezing the indicator. Then adding the frozen indicator to the flask and letting it melt before titrating. Freezing was mentioned in one of the first comments on compound chem's page. But I've never tried it.
Reply to this comment...
Log in to comment
The research shows that natural titration indicators, with good shelf life, do exist ( see Hippeastrum, above). However, Red cabbage does not appear to be one of these indicators.
Reply to this comment...
Log in to comment
The data shows that natural titration indicators with long shelf life (90 days) do exist ( see the Hippeastrum note above). However, Red cabbage does not appear to fit in this category.
Reply to this comment...
Log in to comment
Login to comment.