October 05, 2022 13:17 / Last edited by coremanoma over 2 years ago
New Oral Testosterone Undecanoate Formulation - Another Oral Testosterone Undecanoate OK'd for Hypogonadism
New Oral Testosterone Undecanoate Formulation - Another Oral Testosterone Undecanoate OK'd for Hypogonadism
This androgen is indicated for testosterone replacement therapy in adult men for conditions associated with a deficiency or absence of endogenous testosterone, including congenital or
🌡 Injectables AAS / Oral AAS / HGH / Weight Loss / Peptides / Post Cycle Ttherapy
💪 High Quality / Secured Payment / Guaranteed Confidentiality / Private Data Protection
🥇 Customer support / International shipping / Secure & private
🏉 CLICK HERE TO SHOP ONLINE: https://t.co/AaeClMVKF8
JATENZO is the first FDA-approved oral softgel formulation of testosterone undecanoate used to treat patients with a deficiency or absence of endogenous Newmark J, Stark S, et Real-World Experience with First FDA-Approved Oral Testosterone Undecanoate Paper presented at: 2021 Sexual Medicine Society of
Oral testosterone undecanoate shows promising results for first use
In this video, Jay Newmark, MD, MBA, discusses the background, findings, and takeaways of the study, "Real-World Experience with First FDA-Approved Oral Testosterone Undecanoate Formulation," presented recently at the 2021 Sexual Medicine Society of North America Fall Scientific
FDA Approves Oral Testosterone Replacement Therapy Tlando
Tlando is supplied as5mg of testosterone undecanoate capsules; it is not substitutable with other oral testosterone undecanoate
A new oral testosterone (TLANDO) treatment regimen without
TLANDO restored testosterone levels to the normal range in the majority of hypogonadal This new oral testosterone replacement therapy can provide an option for no-titration oral testosterone replacement This therapy has the potential to improve patient compliance in testosterone replacement 1 INTRODUCTION
Antares Pharma Announces FDA Approval Of TLANDO(tm), an Oral
ewing,, march 29, 2022 (globe newswire) -- antares pharma, (nasdaq: atrs) (the "company"), a specialty pharmaceutical company, today announced that the food and drug
FDA Approves Oral Testosterone Replacement Therapy for Hypogonadism
The FDA has approved the oral drug estosterone undecanoate (Tlando; Antares Pharma, Inc) for testosterone replacement therapy (TRT) for conditions associated with a deficiency or absence of endogenous testosterone, or hypogonadism in adult "The FDA approval of Tlando brings to market an oral formulation of testosterone that
Oral Testosterone | SpringerLink
For years, oral formulations were elusive due to poor efficacy and However, Jatenzo (oral testosterone undecanoate) obtained FDA approval in Oral testosterone is an appealing option due to its convenience and ease of administration when compared to alternate
A new oral T (TLANDO) treatment regimen without dose
TLANDO is an oral capsule product having5 mg of TU in a unique lipid formulation containing predominantly predigested triglycerides (mono- or di-glycerides) It was designed to enable absorption of TU via the intestinal lymphatic
Inside Patient Care - Aveed (Testosterone Undecanoate): A Novel
Testosterone undecanoate is a long-acting depot formulation of testosterone formulated in refined castor oil and benzyl 10 The new drug has a novel dosing schedule: a single intramuscular (IM) injection is given initially, followed by a second injection 4 weeks Subsequent injections are given every 10 weeks10
Testosterone Replacement Therapy: A Narrative Review with a Focus
TLANDO (r) (Lipocine, Salt Lake City, UT, USA), another oral formulation of TU using SEDDS, has recently been approved by the FDA for the treatment of male 44 As this formulation does not require dose titration, patients do not need to have regular blood work, making use and follow-up
Cureus | Is Oral Testosterone the New Frontier of
Testosterone administration for the treatment of hypogonadism routinely consists of either transdermal application of specially formulated gels, intramuscular/subcutaneous injections, or oral tablets taken by Recently, the United States FDA approved the use of oral testosterone undecanoate in treating men with certain forms of
FDA approves Tlando an oral testosterone deficiency treatment
Published: 30th Mar 2022 Antares Pharma,, a specialty pharmaceutical company, announced that the FDA granted final approval for Tlando (testosterone undecanoate), an oral treatment for testosterone replacement therapy indicated for conditions associated with a deficiency or absence of endogenous testosterone, or hypogonadism in adult
Clarus Therapeutics to Present New Real-World Experience
Abstract Title: "Real-World Experience with First FDA-Approved Oral Testosterone Undecanoate Formulation" Date/time: Thursday, October 21, 2021; 4:20 PM MST Session: Androgens Abstract Session #2
Testosterone Replacement Options
New oral testosterone therapy offers men with certain forms of hypogonadism a novel formulation that reduces the dietary-fat effect and variability of testosterone response observed with previous oral Oral testosterone undecanoate, approved by the FDA is the first new oral testosterone replacement product in more than 60
FDA Approves Oral Testosterone Replacement Therapy for
The FDA has approved the oral drug estosterone undecanoate (Tlando; Antares Pharma, Inc) for testosterone replacement therapy (TRT) for conditions associated with a deficiency or absence of endogenous testosterone, or hypogonadism in adult
Regulatory update on oral native testosterone - Diurnal
Native oral testosterone (DNL-0300 previously referred to as DITEST(tm)) is a novel formulation developed by Diurnal comprising native testosterone adapted for oral delivery for the treatment of
Is Oral Testosterone The New Frontier Of Testosterone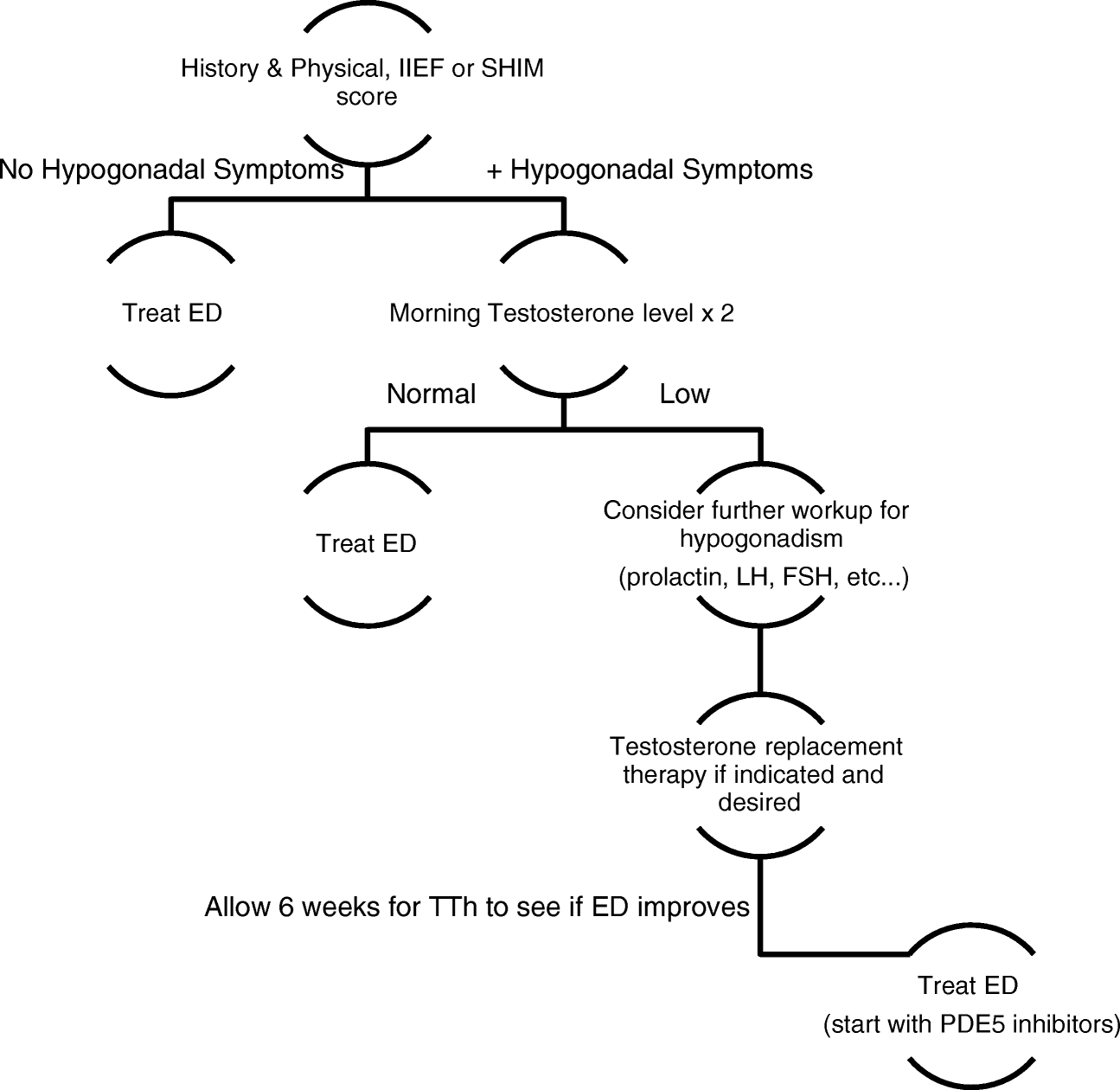
The oral testosterone undecanoate group started the study with a dose of 237 mg of testosterone undecanoate twice a day prior to breakfast and dinner to allow for a 12-hour Based on average testosterone levels measured on days 21 and 56 of the study, doses could be changed to 316 mg and then 396 mg or decreased to 198mg and 158mg
Clarus Therapeutics Announces Issuance of Two New Patents for
JATENZO is the first and only FDA-approved oral softgel for testosterone replacement therapy (TRT) in adult males who have deficient testosterone due to certain medical conditions Clarus plans to list these patents in FDA's Orange Book, which would bring the total number of Orange Book-listed patents covering JATENZO to seven
Oral Testosterone | Request PDF
Context A novel formulation of oral testosterone (T) undecanoate (TU) was evaluated in a phase 3 clinical Objective Determine efficacy, short-term safety, and alignment of new oral->Previous Page - Next Page<-